Download Drug Screen Template
The Drug Screen form is a crucial document used in the process of drug testing, ensuring that all necessary information is accurately captured and maintained throughout the testing procedure. This form begins with essential details about the employer and the medical review officer (MRO), including their names, addresses, and contact information. It also requires the donor's Social Security Number or employee identification number, which helps in tracking the specimen. The form specifies the testing authority, such as HHS or DOT, and outlines the reason for the test, whether it be pre-employment, random, or due to reasonable suspicion. Additionally, it lists the types of drug tests to be performed, such as THC, cocaine, and others, providing clarity on what substances are being screened. The form includes sections for the collector to record observations during the collection process, ensuring that the specimen's integrity is maintained. Following the collection, a chain of custody is established, detailing how the specimen is handled and transported to the testing facility. The results section indicates whether the specimen tested negative or positive for various substances, and provides a space for remarks if the specimen is rejected or if there are any issues during testing. Each step is designed to uphold the highest standards of accuracy and compliance with federal requirements, making the Drug Screen form a vital part of the drug testing process.
Key takeaways
When it comes to filling out and using the Drug Screen form, there are several important considerations to keep in mind. Here are key takeaways that can help ensure the process runs smoothly:
- Accurate Information is Essential: Ensure that all fields, including employer details, donor identification, and testing authority, are filled out accurately. Incomplete or incorrect information can lead to delays or invalid results.
- Understand the Testing Authority: Familiarize yourself with the various testing authorities listed on the form, such as HHS, NRC, and DOT. Knowing which authority governs your testing can help clarify the specific requirements that must be met.
- Specify the Reason for Testing: Clearly indicate the reason for the test, whether it is pre-employment, random, or due to reasonable suspicion. This context is crucial for the testing process.
- Follow Chain of Custody Procedures: The chain of custody is vital for maintaining the integrity of the specimen. Ensure that all steps, from collection to laboratory receipt, are documented accurately and that seals are affixed properly.
- Temperature Check is Critical: The collector must read the specimen temperature within four minutes. A temperature between 90° and 100° F is required to ensure the sample is valid. Any discrepancies should be noted in the remarks section.
- Document Observations: If the collection is observed, or if there are any issues during the collection process, these should be recorded in the remarks section. Transparency is key in the testing process.
- Review Results Thoroughly: After testing, results will be reported as negative, positive, or rejected. Carefully review these results, as well as any remarks provided by the testing facility, to understand the outcome fully.
By adhering to these guidelines, you can help ensure that the drug screening process is efficient, compliant, and effective.
Guide to Writing Drug Screen
Filling out the Drug Screen form is a straightforward process that ensures accurate documentation and compliance with federal regulations. Each step requires careful attention to detail to ensure that all necessary information is recorded properly. Follow the steps below to complete the form effectively.
- Step 1: Complete the section designated for the collector or employer representative. This includes providing the following information:
- Employer Name, Address, and I.D. Number
- MRO Name, Address, Phone, and Fax Number
- Donor SSN or Employee I.D. Number
- Specify Testing Authority (HHS, NRC, DOT) and DOT Agency if applicable
- Reason for Test (Pre-employment, Random, etc.)
- Drug Tests to be Performed (THC, COC, etc.)
- Collection Site Name, Code, Address, and Collector’s Contact Information
- Step 2: The collector should read the specimen temperature within 4 minutes. Indicate if the temperature is between 90° and 100° F and make remarks as necessary. Specify whether the collection was split, single, or none provided, and note any observations.
- Step 3: The collector needs to affix bottle seals to the specimen bottles, date the seals, and have the donor initial the seals. The donor must also complete Step 5 on Copy 2 (MRO Copy).
- Step 4: Initiate the chain of custody. The collector certifies that the specimen was collected, labeled, sealed, and released in accordance with federal requirements. Fill out the details of the delivery service and collect signatures from both the collector and the accessioner.
- Step 5A: The test facility completes the primary specimen report. Indicate whether the result is negative or positive for specific substances and note any remarks. The certifying scientist must sign and date this section.
- Step 5B: If applicable, the split testing laboratory will confirm or fail to reconfirm the specimen. Provide reasons if the specimen failed to reconfirm, and the certifying scientist must sign and date this section as well.
By following these steps, you ensure that the Drug Screen form is filled out accurately and completely, which is crucial for maintaining compliance and integrity throughout the testing process.
Browse Other PDFs
Dd Form 2656 March 2022 Fillable - Each service member's retirement journey begins with the submission of the DD 2656.
An Emotional Support Animal Letter form serves as a formal document prescribed by a licensed mental health professional. This letter certifies that an individual requires the companionship of an emotional support animal (ESA) as part of their mental health or psychiatric treatment. The significance of this document lies in its power to grant legal protections to individuals and their ESAs under certain regulations, making it essential to obtain one from a credible source like OnlineLawDocs.com.
Spa Facial Consent Form - The consent form helps establish a basis for informed decision-making by the client.
Form Preview Example
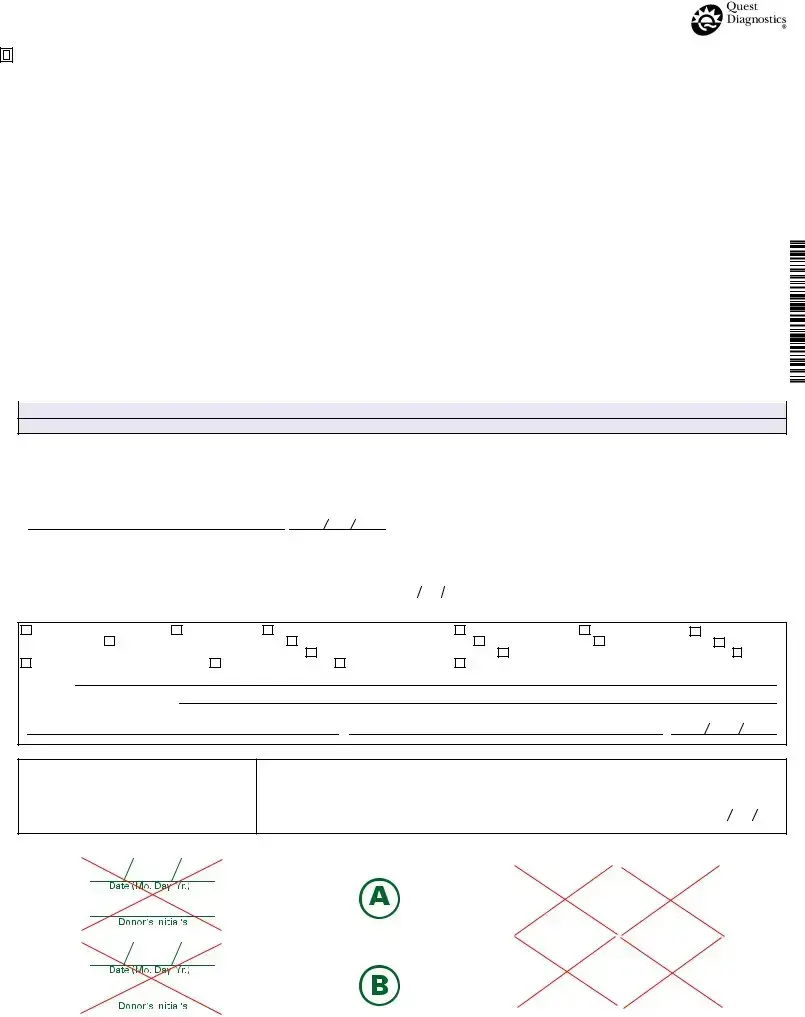
FEDERAL DRUG TESTING CUSTODY AND CONTROL FORM
SPECIMEN ID NO. |
|
STEP 1: COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE |
LAB ACCESSION NO. |
Quest, Quest Diagnostics, the associated logo and all associated Quest Diagnostics marks are the trademarks of Quest Diagnostics Incorporated. © Quest Diagnostics Incorporated. All rights reserved.
A. Employer Name, Address, I.D. No. |
|
|
B. MRO Name, Address, Phone and Fax No. |
||||||||||
|
|
|
|
|
|
|
|
|
|||||
C. Donor SSN or Employee I.D. No. _______________________________________________________________ |
|
|
|
|
|||||||||
D. SpecifyTesting Authority: HHS |
NRC |
DOT – Specify DOT Agency: FMCSA |
FAA |
FRA FTA PHMSA USCG |
|||||||||
E. Reason forTest: |
Random |
Reasonable Suspicion Cause Post Accident |
Return to Duty |
|
|||||||||
F. DrugTests to be Performed: |
THC, COC, PCP, OPI, AMP |
THC & COC Only |
Other (specify) ________________________________________________ |
||||||||||
G. Collection Site Name: |
|
|
|
|
|
Collection Site Code: |
|
|
|
|
|||
Address: |
|
|
|
|
|
|
Collector Phone No.: |
|
|
||||
City, State and Zip: |
|
|
|
|
|
Collector Fax No.: |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STEP 2: COMPLETED BY COLLECTOR (make remarks when appropriate) Collector reads specimen temperature within 4 minutes.
Temperature between 90° and 100° F? Yes No, Enter Remark |
Collection: Split Single None Provided, Enter Remark |
Observed, (Enter Remark) |
REMARKS
STEP 3: Collector affixes bottle seal(s) to bottle(s). Collector dates seal(s). Donor initials seal(s). Donor completes STEP 5 on Copy 2 (MRO Copy)
STEP 4: CHAIN OF CUSTODY - INITIATED BY COLLECTOR AND COMPLETED BY TEST FACILITY
|
I certify that the specimen given to me by the donor identified in the certification section on Copy 2 of this form was |
|
SPECIMEN BOTTLE(S) RELEASED TO: |
|||||||
|
collected, labeled, sealed, and released to the Delivery Service noted in accordance with applicable Federal requirements. |
Quest Diagnostics Courier |
||||||||
|
|
X |
|
|
|
|
|
FedEx |
||
|
|
Signature of Collector |
|
|
|
|
|
Other |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AM |
|
|
|
|
|
|
|
|
|
|
PM |
|
|
|
|
|
|
(Print) Collector's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|
Time of Collection |
|
|
Name of Delivery Service |
||
RECEIVED AT LAB OR IITF: |
|
|
|
|
|
Primary Specimen |
SPECIMEN BOTTLE(S) RELEASED TO: |
|||
|
X |
|
|
|
|
|
Bottle Seal Intact |
|
||
|
|
|
|
|
|
Yes No |
|
|||
|
|
Signature of Accessioner |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If No, Enter remarks |
|
|
|
|
|
|
|
|
|
|
in Step 5A. |
|
|
|
|
(Print) Accessioner’s Name (First, MI, Last) |
|
|
|
Date (Mo./Day/Yr.) |
|
|||
STEP 5A: PRIMARY SPECIMEN REPORT - COMPLETED BY TEST FACILITY
NEGATIVE |
POSITIVE for: |
Marijuana Metabolite ( |
6- Acetylmorphine |
Methamphetamine |
MDMA |
|
DILUTE |
|
|
Cocaine Metabolite (BZE) |
Morphine |
Amphetamine |
MDA |
|
|
|
PCP |
Codeine |
|
MDEA |
REJECTED FOR TESTING |
ADULTERATED |
SUBSTITUTED |
INVALID RESULT |
|
|
|
REMARKS:
Test Facility (if different from above):
I certify that the specimen identified on this form was examined upon receipt, handled using chain of custody procedures, analyzed, and reported in accordance with applicable Federal requirements.
X
Signature of Certifying Scientist |
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
STEP 5b: COMPLETED BY SPLIT TESTING LABORATORY
RECONFIRMED FAILED TO RECONFIRM - REASON ____________________________________________
___________________________________________ |
I certify that the split specimen identified on this form was examined upon receipt, handled using chain of custody |
||||||||||||||||||||||||||||
procedures, analyzed and reported in accordance with applicable Federal requirements. |
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Name |
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
___________________________________________ |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Signature of Certifying Scientist |
|
|
|
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Address |
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMB No.
PRESS HARD - YOU ARE MAKING MULTIPLE COPIES
Documents used along the form
When conducting drug screenings, various forms and documents accompany the Drug Screen form to ensure compliance, maintain proper records, and facilitate communication between involved parties. Each document plays a vital role in the drug testing process, helping to protect the rights of the donor while ensuring that the testing is conducted fairly and accurately.
- Chain of Custody Form: This document tracks the handling of the specimen from collection to testing. It ensures that the sample remains uncontaminated and that all individuals who handle the sample are documented.
- Consent Form: This form is signed by the donor, granting permission for the drug test to be conducted. It outlines the rights of the donor and the procedures involved in the testing process.
- Laboratory Results Report: This report provides the findings of the drug test, detailing any substances detected in the specimen. It is critical for employers and Medical Review Officers (MROs) to make informed decisions.
- Doctor's Excuse Note: In cases where an employee must miss work due to medical reasons, a Doctor's Excuse Note serves as an essential document. It verifies the legitimacy of the absence and can be acquired easily through resources like https://smarttemplates.net/fillable-doctors-excuse-note.
- Medical Review Officer (MRO) Report: After reviewing the laboratory results, the MRO provides a report that includes interpretations of the findings, especially in cases of positive results, considering any medical explanations.
- Donor Identification Form: This form collects essential information about the donor, including their identification details, which helps verify their identity during the testing process.
- Specimen Collection Log: This log records the details of the specimen collection process, including the date, time, and collector's information, ensuring transparency and accountability.
- Testing Authority Notification: This document notifies relevant authorities about the testing process, especially in regulated industries, ensuring compliance with federal or state laws.
- Follow-Up Testing Form: If an initial test is positive, this form is used to request a follow-up test on a split sample, ensuring that results are verified before any actions are taken.
- Employee Handbook Addendum: This addendum outlines the company's drug testing policy, including procedures, consequences for positive tests, and the rights of employees, promoting awareness and understanding.
- Incident Report Form: In cases where drug testing is a result of an incident, this form documents the details of the incident, providing context for the testing and any subsequent actions taken.
Each of these documents is essential in the drug testing process. They work together to create a comprehensive framework that supports the integrity of the testing procedure while safeguarding the rights of all parties involved. Understanding these forms can help ensure a smoother experience for both employers and employees during drug screenings.
